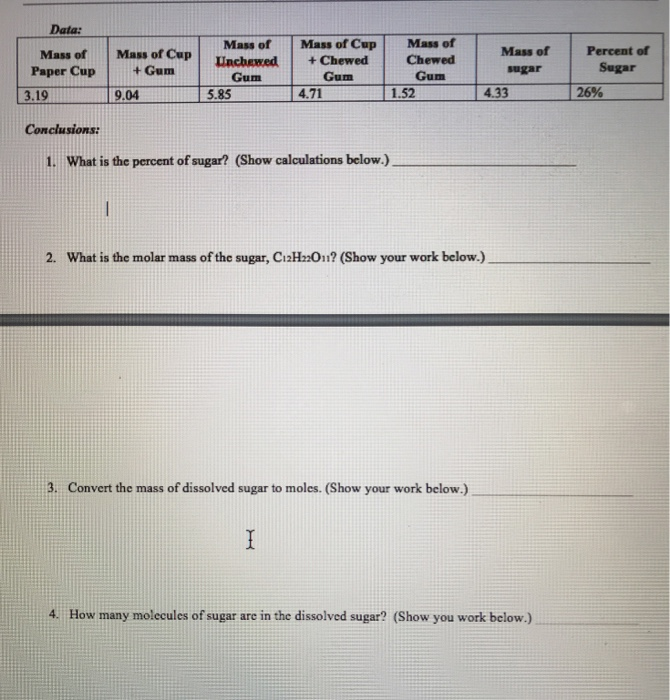
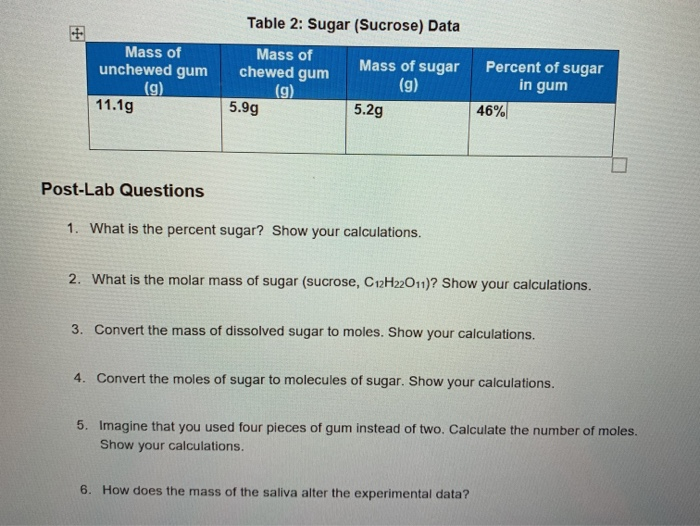
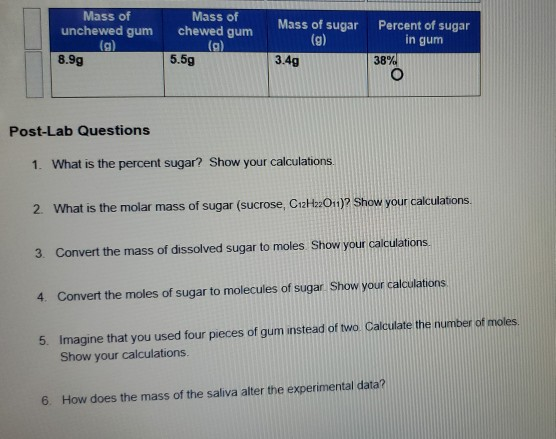
Mass Of Dissolved Sugar To Moles
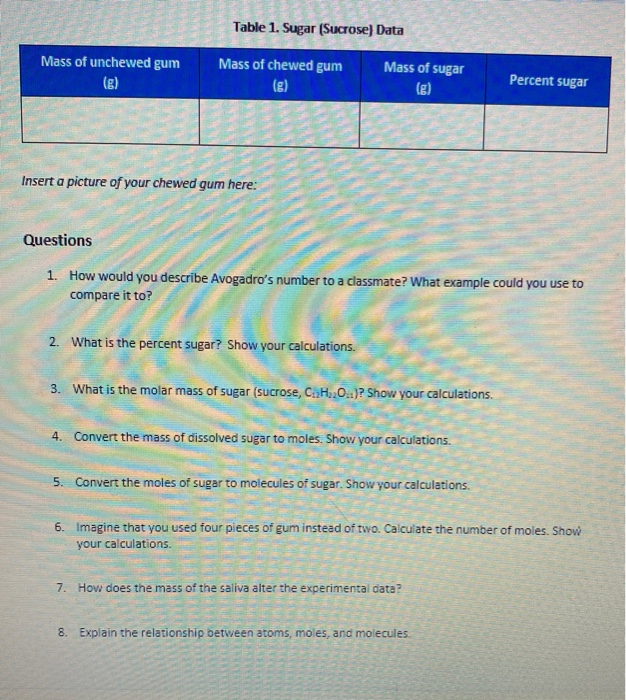
The question states this.
Mass of dissolved sugar to moles. And the most difficult task here is finding out the molar mass of the substance. Considering that sucrose c12h22o11 is being used how many molecules of sugar are there. After the mass in grams is determined the mass will be converted to moles of sugar and molecules of sugar. Sugar water dishwashing detergent steel windscreen washer fluid air.
An explanation of how to use dimensional analysis to find moles when you know the mass. There is a simple relation between these two where mass of the substance in grams quantity of the substance in moles molar mass of the substance in grams mole. Use this example molarity calculation of a sugar solution to practice. The concept of molarity can be tough to grasp but with enough practice you ll be converting mass to moles in no time.
Molality is the number of moles of solute dissolved material per kilogram of solvent. 3 in e flat major op. Convert atoms of sugar to grams of sugar. Table sugar sucrose c12h22o11 molecular mass molar mass type the number of table sugar sucrose c12h22o11 you want to convert in the text box to see the results in the table.
Convert the moles of sugar to molecules of sugar. Molarity is a unit in chemistry that quantifies the concentration of a solution by measuring moles of solute per liter of solution. Convert the mass of dissolved sugar to moles. Do a quick conversion.
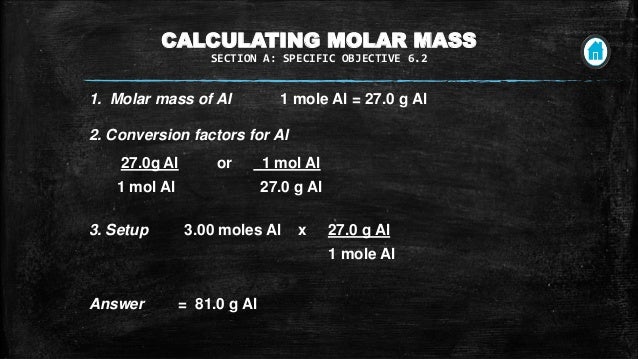
For chemistry you often need to convert moles to grams and grams to moles. 1 grams sugar mole using the molecular weight calculator and the molar mass of sugar. I m aware that you have to convert grams to moles back to molecules but can you please explain and give an answer. Imagine that you used four pieces of gum instead of two.
1 grams c12h22o11 0 0029214440066693 mole using the molecular weight calculator and the molar mass of c12h22o11. The sugar the solute is dissolved in water the solvent. Do a quick conversion. We therefore divide the weight by the molar mass to get moles 1000 18 02 55 5 m.





/pipette-pouring-water-on-sugar-cube-in-spoon-663787601-57fb97c23df78c690f799c5c.jpg)






